
Want to learn more about redox reactions and the type of biological oxidation and reduction MCAT content you’ll encounter on testing day? Look no further!
Below, we’ll cover the core concepts you’ll want to study in order to ace this material on the Chemical and Physical Foundations of Biological Systems section of the MCAT. Also feel free to read more about the other physics and chemistry topics tested on the MCAT!
For a handy PDF version of this biological oxidation and reduction MCAT material, and many other awesome MCAT resources, click the link below!
MCAT Reduction/Oxidation and Potentials
Okay, first things first, let’s get clear on redox reactions and what they are. In redox reactions, charge is transferred between reactants and products in the form of electrons.
However, the best way to understand redox reactions is to understand the significance of electrons and Oxygen in the reaction and oxidation of reagents in reactions.
MCAT Oxidation Numbers
Oxidation numbers are assigned based on conventional guidelines:
- Free Elements (Br2, O2, etc.) are zero.
- Monatomic ions are equal to their ionic charge.
- Group IA and IIA elements are +1, +2, respectively.
- Group VII elements are always -1 except in the presence of a more electronegative element (e.g., OF2, O is +2 and F is -1).
- Hydrogen is +1 in the presence of electronegative elements and -1 in the presence of electropositive elements (H2O vs CH4); likewise for Oxygen.
- In a molecule, the total molecular oxidation number is the sum of the individual oxidation numbers. In a neutral compound, sum = 0. In a polyatomic ion, sum = formal charge.
Redox reaction concepts will most likely come up as biochemical reactions, such as the reduction or oxidation of Plastoquinone, shown below:
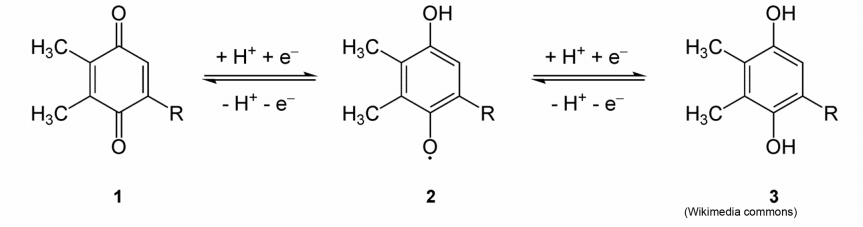
- Note that as the molecule is reduced, protons bind to the oxygen.
- Electrons can usually be followed in a biochemical reaction by following protons.
Important Facts
- Oxidation (Anode): loss of e–
- Reduction (Cathode): gain e–
- Reducing agent: reduces others; gets oxidized
- Oxidizing agent: oxidizes others; gets reduced
A mnemonic device for remembering this = OIL RIG (Oxidation Is Loss, Reduction is Gain).
Balancing Ionic Equations
Steps for Balancing
- Separate core reagents into reduction and oxidation half reactions.
- Balance O2 for each \( \frac 1 2\) rxn — add H2O and H+ for acidic and H2O and OH for basic solutions.
- Balance the charge for each half by adding e–
- Multiply either or both half reactions to cancel out the e–, add the half reactions.
Example
| Basic reaction | MnO4– + I– → I2 + Mn2+ |
| Fully balanced | 2 MnO4– + 16 H+ + 10 I– → 2 Mn2+ + 5 I2 + 8 H2O |
A Final Word About Biological Oxidation and Reduction MCAT Content
When it comes to studying oxidation and reduction in redox reactions, keep in mind that the MCAT will often blend metabolism, chemical gradients, and redox concepts when testing this topic.
This can be a tricky concept that does involve a fair share of memorization, so make sure to review each of the steps thoroughly.
For more MCAT practice, check out Magoosh’s MCAT prep, which includes 380 lessons, 745+ practice questions, personalized email assistance, and more!
Happy studying and good luck!







Leave a Reply