Looking to familiarize yourself with some of the MCAT kinetics content you’ll find on test day? You’ve come to the right place! Below, we’ll cover the key kinetics concepts you’ll want to study including chemical reactions and rates, rate laws, and more. You can also check out our overview of other MCAT physics and chemistry topics here.
For a downloadable PDF version of the MCAT kinetics overview as well as many other great MCAT resources, click the link below!
MCAT Kinetics: Basis of Chemical Reactions and Rates
To start, here are some foundational things you’ll need to know about chemical reaction mechanisms:

Collison Theory of Chemical Kinetics
Reaction rate increases as rate of intermolecular collisions increases.
Collisions increase or decrease due to:
- Reaction concentrations (Le Chatelier’s, Keq)
- Temperature (remember physiological limits!)
- Medium (correct solute)
- Presence of catalysts
Reaction Rates and Rate Laws
MCAT reactions rates are basically the rate of product formation or rate of reactant consumption.
A couple of things to keep in mind when you’re figuring out how to determine rate law:
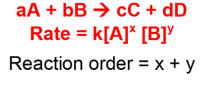
Reaction Orders
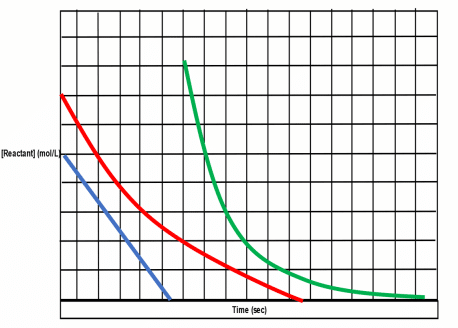
You’ll want to remember the following about reaction orders:
- Zeroth Order Reactions: Reactant concentration will have no effect on rate.
- First Order Reactions: Rate will increase/decrease linearly to reactant concentration. Double reactant = double rate.
- Second Order Reactions: Rate will increase/decrease exponentially to reactant concentration. Double reactant = quadruple rate.
MCAT Kinetics: Final Tips and Reminders
Here’s a helpful reactions shortcut for you to memorize as you’re studying:
Zeroth = Zero Dependents
First = One Dependent
Second = Squared or Two
Also, here are some important things to remember for test day:
- If the passages focus on gen chem, then kinetics questions may be more chem focused.
- If the passage delves into biochemistry, then keep in mind physiological constraints and understand rates with regards to enzyme activity.
Want more MCAT practice? Check out Magoosh’s MCAT prep, which includes 380 lessons, 745+ practice questions, personalized email assistance, and more!
Happy studying and good luck!