Looking to brush up on thermodynamics MCAT concepts? You’ve come to the right place! We’ll cover key concepts including functions, important equations, heat, Gibbs free energy, and more! You can also check out our overview of all of the physics and chemistry concepts tested on the MCAT.
For a PDF version of this material, as well as other great MCAT resources, click the link below.
MCAT Thermodynamics: States, Systems, and Processes
First, you’ll want to consider states and state processes in terms of the Ideal Gas Law, which is as follows:
PV = nRT
- This would include considering pressure vs. volume
- Work would be calculated as area under the curve
- Adiabatic: P = C/V
- Isovolumetric: NA
- Isothermal: P = nRT/V
[threecol_two]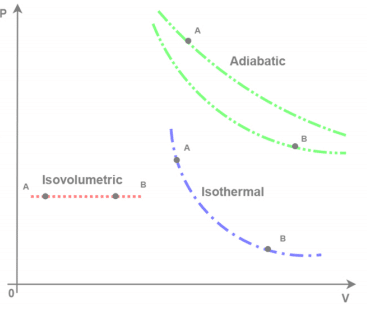 [/threecol_two]
[/threecol_two]
[threecol_one_last]
[/threecol_one_last]
State Functions
States are described as State Functions. Equilibrium is usually measured at Standard conditions or Standard Temperature and Pressure (STP), and equilibrium conditions will be tested on the MCAT.
Standard Conditions: 298K, 1 atm, 1 M concentrations
STP: 273K, 1 atm
Conversion: K = °C + 273
State Functions
- Pressure (P)
- Density (δ)
- Temperature (T)
- Volume (V)
- Enthalpy (H)
- Internal Energy (U)
- Gibbs free energy (G)
- Entropy (S)
Mnemonic device to remember the above: PAT V. HUGS
Newton’s First Law
Newton’s 1st law = △U = Q- W
Change in internal energy = heat added into system – work done by system
Systems
- Isolated: No matter or energy exchange (bomb calorimeters)
- Closed: Energy exchange only (radiators)
- Open: Energy and matter exchange (an open reaction)
Processes
- Adiabatic = no heat exchanged, U =
- Isothermal = T is constant
- Isovolumetric = V is constant = no work
- Isobaric = P is constant
Phase Diagrams
Most relevant reactions will take place in standard conditions so Standard enthalpy, entropy, and free energy values will be used.
Terms to remember:
- Melting vs Freezing (solid ↔ liquid)
- Sublimation vs Deposition (solid ↔ gas)
- Vaporization vs Condensation (liquid ↔ gas)
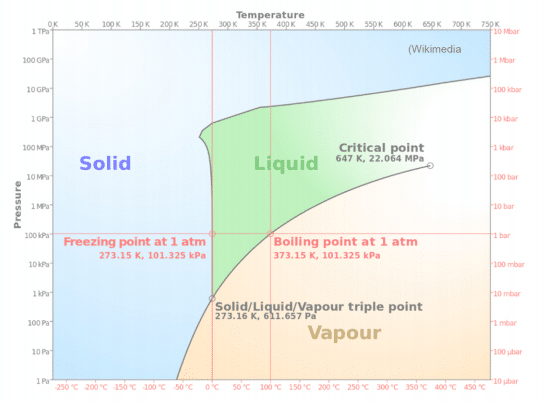
Phase diagram image from Wikimedia Commons
Heat, Enthalpy, Entropy, and Gibbs Free Energy
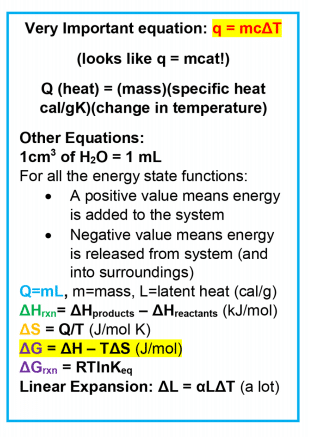
Heat (Q)
Heat (Q) – think of it less as ‘temperature’ and more as the kinetic energy of molecules. Q>0, Q<0 means energy added or removed from system, respectively.
Temperature and heat are both related to the kinetic energy of a substance: heat is the exchange of energy between two substances at different temperatures. Heat is measured using calorimetry.
- Convection: movement in a fluid due to heat transfer, rising of high T fluid, and sinking of low T fluid (gas too)
- Conduction: transfer of heat through direct contact
- Radiation: transfer of heat through electromagnetic waves; infrared radiation
Heating curves — measuring the heat absorbed by a compound as its T rises (may include fusion, vaporization).
Enthalpy (△H)
Enthalpy (△H) — total heat of a compound usually considered with regards to the formation of chemical compounds or processes with a reaction mechanism. Standard enthalpies will be provided.
Entropy (△S)
Entropy (△S) — measure of disorder, but a more accurate definition is the measure of the dispersion of energy.
- 2nd Law of Thermodynamics: Tot energy in a system never decreases, entropy is spontaneously maximized
- Reactions usually have positive entropy
- △Sgasses > △Sliquids > △Ssolids
Gibbs Free Energy (△G)
For MCAT thermodynamics, this is generally considered a measure of the ‘total energy’ in a system or product.
Very important to determine the spontaneity of a reaction:
- Spontaneity has no bearing on the kinetics (speed) of a reaction. This can be a pitfall for some of the comprehensive kinetics and thermodynamics MCAT questions.
- (+)△G means reaction is non-spontaneous
- (-)△G means reaction is spontaneous
| △G | △H | △S | Spontaneous? |
|---|---|---|---|
| (+) at low T, (-) at high T | + | + | Yes, at high T |
| Always (-) | + | – | Never |
| Always (+) | – | + | Always |
| (-) at low T, (+) at high T | – | – | Yes, at low T |
A Final Word About MCT Thermodynamics Concepts
As far as thermodynamics MCAT content goes (and really all MCAT content!), remember that the MCAT tests your understanding more than it tests your ability to plug and chug equations. Knowing units and having strong dimensional analysis skills will make answering MCAT thermodynamics questions much easier.
Also, keep in mind that many times, the questions and answers will have hints in the form of units or state functions used that will partially answer the question for you.
Finally, for more MCAT practice, check out Magoosh’s MCAT prep, which includes 380 lessons, 745+ practice questions, personalized email assistance, and more!
Happy studying and good luck!