
The MCAT is going to test you on aromatics, so it’s a good idea to start brushing up on your organic chemistry as soon as possible.
Ready to get started? Check out our free video lesson on MCAT Aromatics, and keep reading for a quick aromatics review.
MCAT Aromatics Review
To determine if a molecule is aromatic, we need to consider three important factors.
- It must have alternating double and single bonds, which are arranged into a cyclical structure or a ring.
- If there are multiple rings, they need to be coplanar (meaning they need to be flat).
- They must obey Huckel’s Rule, which says they will have 4n+2 pi electrons. This means that they can have 2, 6, 10, 14, 18, etc., electrons.
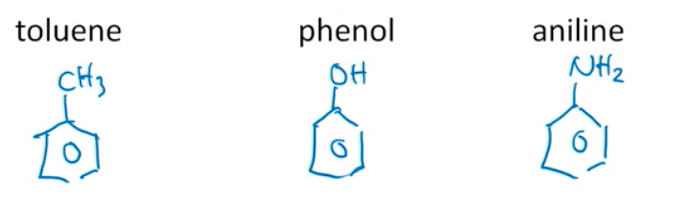
Aryl Compounds to Know:
There are a couple of common aryl compounds you want to have memorized:
- Toluene is a benzene ring with a methyl group attached to it.
- Phenol is a benzene ring with a hydroxyl group attached to it.
- Aniline is a benzene ring with a NH2, or amino group attached to it.
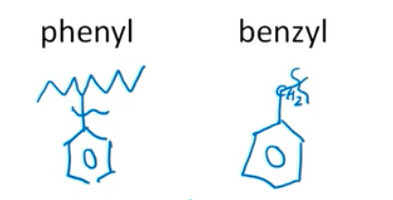
Common Aryl Substituents to Know:
- Phenyl group: when we have direct attachment of the benzene ring.
- Benzyl group: when there’s a methyl group between the attachment.
Prefixes to Know:
Now, in the case of disubstituted rings, there are several prefixes you’ll want to memorize.
- Ortho- as a prefix indicates substitution on adjacent carbons.
- Meta- as a prefix indicates one empty between the substituted carbons and
- Para- as a prefix indicates the substitutions are on opposite sides of the ring.
Multiple Substitutions:
So far, we’ve only talked about a single substitution to a ring, but if we have multiple substituents, each substituent will direct subsequent editions.
There are three basic types:
- Activating ortho/para: these are electron donating substituents. This includes NH2, NR2, And R alcohol groups. Essentially, these are all groups with lots of electrons that they can donate.
- Deactivating ortho/para: these are weakly electron withdrawing substituents. This includes the halogens, these are electronegative species that have some affinity to electrons and so pull it away from the rim.
- Deactivating meta: these are strongly electron withdrawing substituents that include things like NO2, SO3H, Carboxylic acid, Ester, Ketone and Aldehyde. So these things tend to have strong resonance structures so they can deal localized electrons. For example, the carboxylic acid.
More MCAT Study Materials:
Are you on the market for an MCAT study schedule? More free MCAT lessons? Maybe this MCAT Aromatics review was less of a “review” than you’d hoped…don’t worry! Our official MCAT test prep has you covered.
Whatever you’re looking for, check out the links below for everything MCAT:



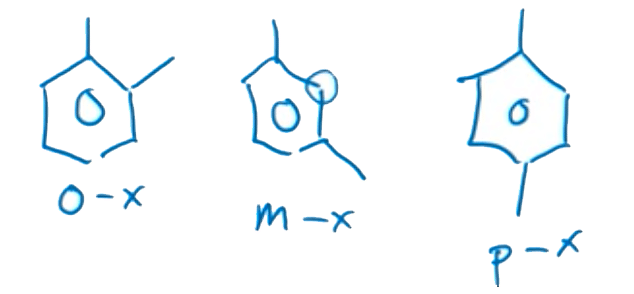

Leave a Reply