One type of ACT Science passage, Research Summaries, will present descriptions of one or more related experiments and will require you to answer questions about one or both experiments. To compare them accurately, answer the following questions as you read:
How is each experiment set up? Make sure you understand the method for each experiment. What tools/processes/chemicals are used?
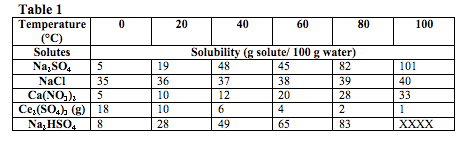
What does the data show? Pay close attention to the results of each experiment. Usually this is presented in tables or charts. How do the different variables relate to one another? Draw arrows on the tables to show the trends.
How do the experiments differ? There will be certain elements common to both experiments, and one or more elements will change from Experiment 1 to Experiment 2. Circle the new information. Then focus on the results – do the variables interact similarly or differently in the 2nd experiment? Is the range of data greater or smaller?
Try a sample passage and practice question!
Passage I
A student wishes to discover for herself what effects, if any, pressure and temperature have on the solubility of various solutes in water. The solute of a solution is the substance that is dissolved by the solvent.
Experiment 1
At a constant pressure of 770 torr, a student tested the solubility of five compounds at different temperatures. In order to do this, the student started with 100g of water and gradually added the solutes, in five separate trials, until no more of that solute could dissolve. All of the solutes tested in this experiment were in the solid state unless otherwise indicated. The results of this experiment are summarized in Table 1.
Experiment 2
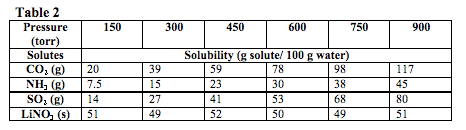
A second experiment was conducted at a constant temperature of 25˚C under different pressures. In order to do this, the student gradually increased the pressure of the solutions while determining the effect on solubility. The solvents tested were in either solid or liquid state. The results of this experiment are summarized in Table 2.
Question
The solubility of CO2 is tested at 25° and 75 torr. According to the data, its solubility in g solute/100 g of water is most likely closest to what value?
(A) 5
(B) 10
(C) 45
(D) 50
According to Table 2, the pressure and solubility of a gas are directly proportional. Since the solubility of CO2 is 20 g/100 g water at 150 torr, its solubility at 75 torr should be half of 20, or 10. The correct answer is (B).






Leave a Reply